
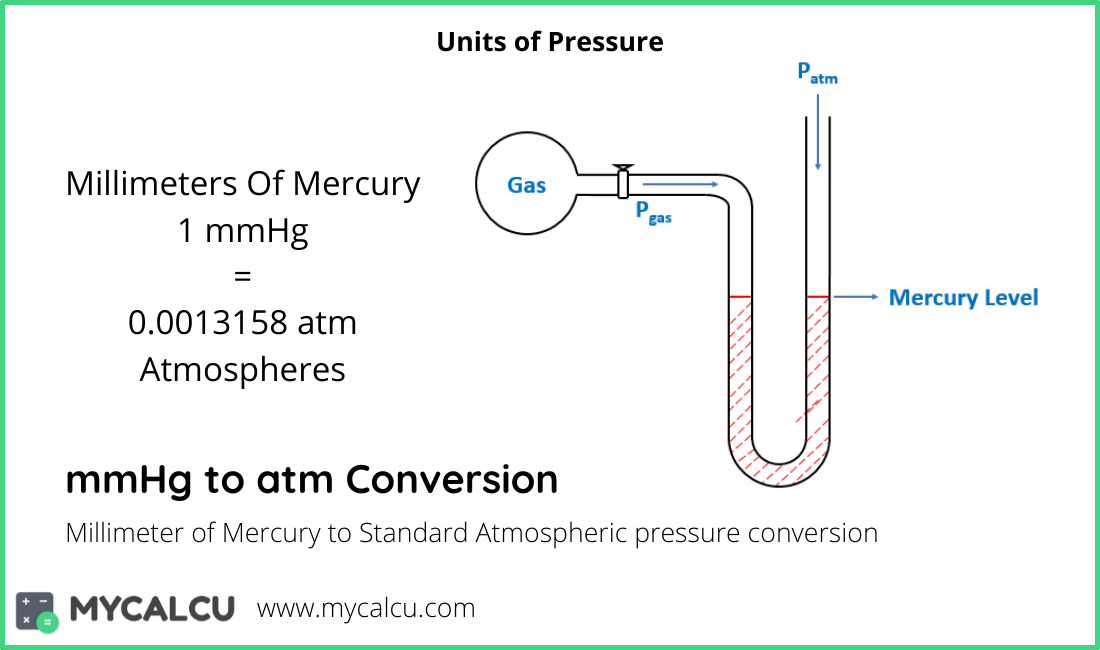
Below, figures and tables show the boiling point of water at varying vacuum. Note Pressure must be within the ranges 0-1000 mbara, 0-14.7 psia, 0-760 mm Hg or 0-30 in Hg. 739 standard atmospheres are equal to 7.29336 kilopascals. The pressure p in pascal (Pa) is equal to the pressure p in millimeter mercury (0°c) (mmHg) times 133.32237, that conversion formula: p(Pa) p(mmHg) × 133.
739 MMHG TO ATM HOW TO
How to convert Millimeter Mercury (0☌) to Pascal 1 millimeter mercury (mmHg) is equal to 133.32237 pascal (Pa). The output temperature is given as ☌, ☏, K and °R. Learn how to convert from kilopascals to atm and what is the conversion factor as well as the conversion formula. 739 Millimeter Mercury (0☌) 98525.23026 Pascal. Let’s see some interconversion from 1 atm. The calculator below can be used to calculate the water boiling point at given, absolute pressures. 1 atm 760 mmHg For the second conversion, 1 atm 101.325 kPa. Note: We know the interconversion between the units to do various calculation problems. CHAPTER 5 GASES 5.13 1 atm 562 mmHg 760 mmHg 0.739 atm 5.14 Strategy: Because 1 atm 760 mmHg, the following conversion factor is needed to obtain the pressure in atmospheres. So we can expresses the equation of pressure as, We know that pressure is the force exerted in a specific area and it is calculated by dividing the force applied by the area on which the force was applied. If you would like help with converting between mmHg and atm, give our mmHg. This means that to convert mmHg to atm you should multiply your figure by 0.0013157896611399.

One millimeter of mercury is equal to 0.0013157896611399 atmospheres. So before going into the solution part of the question, let’s discuss some concepts about pressure and its unit. The atmosphere (atm) unit is commonly used for referencing the average atmospheric pressure at sea level. 3.0 atm, to the waters surface, where the temperature is 25C and the pressure. In the question, it is asked that we have to convert the given value of unit in mm Hg to atmospheres of pressure.The question is simply about te unit conversion of values between the units of pressure, A sample of hydrogen gas was collected over water at 21C and 685 mmHg. We should be familiar with 1 atm value equal to how much value of mmHg. Hint: The approach for the question should be done by focusing on the unit conversion of the value from mmHg to atmospheres of pressure in atm.


 0 kommentar(er)
0 kommentar(er)
